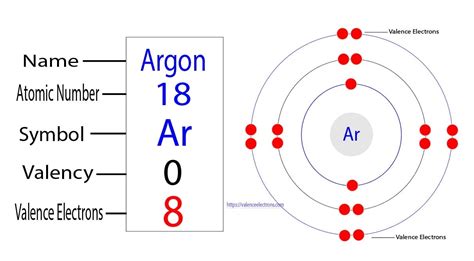
valence electrons argon|transition metals valence electrons list : Clark Well, Argon has an absolute zero valency. Argon holds 18 electrons in its outer shell with the electron configuration of 2,8,8. So, this is why Argon has no need to . If you have questions, concerns, or feedback to share about paying online through grpayit, please get in touch using the "Hi.Need any help?" widget in the bottom corner of the screen. For all other questions about your City of Grand Rapids, MI accounts, please contact the City of Grand Rapids directly by dialing 311 from a local line, or call 616-456-3000.
valence electrons argon,Mar 23, 2023

Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p . You may assume the valences of the chemical elements—the number of . The total number of electrons in the last shell after the electron configuration of argon is called the valence electrons of argon. The last shell of argon has eight .
Well, Argon has an absolute zero valency. Argon holds 18 electrons in its outer shell with the electron configuration of 2,8,8. So, this is why Argon has no need to . The total number of electrons present in the valence shell of an atom is called valence electrons, and there are eight electrons present in the valence shell of argon (3s²3p⁶). Thus, argon has eight .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .
Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, .In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Now, the electron configuration of argon shows that the last orbit has eight electrons. Therefore, the valence electrons of argon are eight. The last shell of argon has no unpaired electron, so the . La configuration électronique de l’argon montre que sa dernière couche a huit (3s 2 3p 6 ) électrons. Les électrons de valence dans l’argon (Ar) en ont huit. La valence est une caractéristique . The \(1s\) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the \(2s^22p^4\) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation \(\ref{3}\)), the electrons in the argon-like closed shell are the core . 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. There are two ways to find the number of valence electrons in Argon (Ar). The first is to use the Periodic Table to figure out how many electrons Argon has i.
Examples include hydrogen (H), lithium (Li), and sodium (Na). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, like all the other elements in group 16, has six valence electrons. Discuss further with Flexi.
This is why third-row elements, such as argon, can be stable with just eight valence electrons: their s and p subshells are filled, even though the entire 3n shell is not. While electron shells and orbitals are closely related, orbitals provide a more accurate picture of the electron configuration of an atom. Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .valence electrons argonWhen we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital.How to determine the number of valence electrons and draw Lewis structures for main group elements starting from the electron configuration. Created by Jay. . then chlorine would have an electron configuration like a noble gas, like that of argon. So chlorine will gain an electron here. So let's go ahead and write the new electron .

As a result, the electron configuration for argon will be \(1s^22s^22p^63s^23p^6\) Because the third energy level having eight electrons and is therefore it is full \(3s^23p^6\)it is called a noble gas. Valency of Argon. Argon has 8 valence electrons in its outermost shell. So it will neither lose any electron nor gain .valence electrons argon transition metals valence electrons listElement Argon (Ar), Group 18, Atomic Number 18, p-block, Mass 39.95. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element . Argon has 8 valence electrons in its outer shell. Argon Electron Configuration Excited State. Electron configuration of Ar is 1s2 2s2 2p6 3s2 3p6. Filed Under: Period Table. This site provides the Argon Electron Configuration (Ar) with Orbital Diagram and the position of Argon in the periodic table chart.transition metals valence electrons list For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic . Explanation: Argon is a noble gas with an electron configuration of [N e]3s23p6. So, it has 3 electron shells, and that will be its valence shell. In the third shell, it holds a total of 2 + 6 = 8 electrons, so it has 8 valence electrons. Answer link. 8 Argon is a noble gas with an electron configuration of [Ne]3s^2\3p^6.
Hint: Electrons which are present in the outermost shell of the atom are known as valence electrons and for the calculation of the number of valence shell electrons we should know about the electronic configuration of the atom. Complete Solution : Symbolically Argon is denoted as ‘${\text{Ar}}$’ in the periodic table and its .L'argon possède 26 isotopes connus, du 29Ar au 54Ar et 1 isomère (32 mAr), dont trois stables (36Ar, 38Ar et 40Ar). L'argon-40 est composé de 18 protons, 22 neutrons et 18 électrons. . Cette coque à valence complète rend l’argon très stable et extrêmement résistant à la liaison avec d’autres éléments.
valence electrons argon|transition metals valence electrons list
PH0 · valence electrons for kids
PH1 · valence electrons chart
PH2 · valence electrons calculator
PH3 · transition metals valence electrons list
PH4 · number of valence electrons fe
PH5 · list of valence electrons for each element
PH6 · how do you find valence electrons
PH7 · finding valence electrons in ions
PH8 · Iba pa